Research Overview—
Professor Tolbert’s research focuses on the effects of glycosylation on protein therapeutics and site-selective modification of proteins. Our interest in these topics stem from the fact that post-translational modifications of proteins can alter protein biological activity and stability and can also be used to produce protein drug conjugates.
Studies of the effects of glycosylation on protein therapeutics
Approximately 50% of FDA approved protein therapeutics are glycosylated, and this modification can have significant effects on protein activity and stability. In addition, since protein glycosylation is a heterogeneous modification where one protein may have many different oligosaccharides attached to it, glycosylated protein therapeutics are not just single proteins defined by their amino acid sequences, but a mixture of glycosylated protein forms (glycoforms). The mixtures of glycoforms that make up glycosylated protein therapeutics can have a range of biological activities, stabilities and in vivo half-lives and this complicates production and analysis of these types of therapeutics.
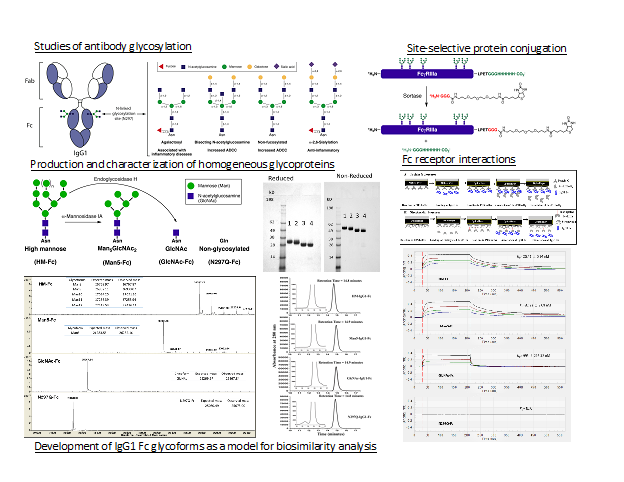
To better understand the effects of glycosylation on protein therapeutics we study antibody glycosylation. Monoclonal antibodies based on the human IgG class are the largest category of approved protein therapeutics. Glycosylation in the fragment crystallizable (Fc) region of the human IgG class of antibodies alters antibody dependent immune responses, stability and half-life. To avoid working with heterogeneous glycoform mixtures, expression in glycosylation deficient yeast and in vitro enzymatic synthesis is used to produce single homogeneous IgG Fc glycoforms. These homogenous glycoforms are used to study the effects of glycosylation on Fc receptor interactions important in immune responses and on the structure and stability of the four human IgG subclasses. In addition, well-defined mixtures of these pure glycoforms have been used to produce model systems to study the effects of glycosylation heterogeneity on biosimilarity and comparability studies.
Site-selective protein modification
Conjugation of drugs and other types of modifications onto therapeutic proteins can produce chimeric molecules with unique properties such as the drug targeting capability of antibody drug conjugates. Unfortunately, many current therapeutic protein-drug conjugates utilize random conjugation chemistry that produces heterogeneous mixtures of drug-protein conjugates. This complicates production and analysis of protein-drug conjugates in a manner similar to the heterogeneity of glycosylated protein therapeutics. We are interested in developing and applying site-selective protein conjugation techniques to the production of well defined, homogeneous therapeutic protein-drug conjugates.
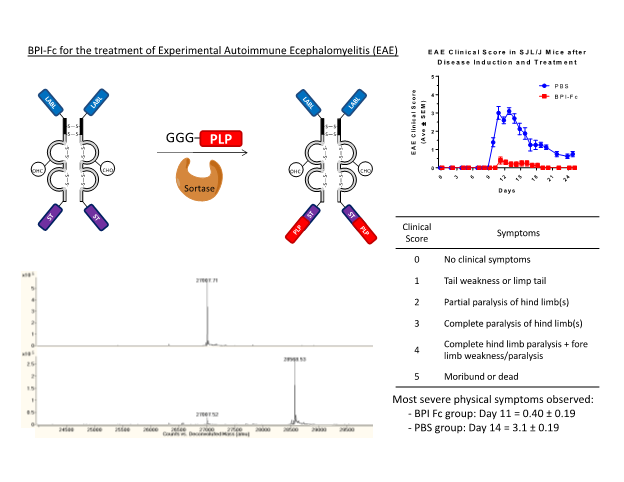
To produce site-selectively modified proteins we develop and utilize chemoselective or enzymatic ligation techniques to specifically attach drugs or peptides to proteins. Our studies have used IgG1 Fc as a carrier protein that has a long in vivo half-life and the potential for engaging Fc receptors involved in immune responses. Native chemical ligation has been utilized to selectively ligate cyclic-RGD peptides to the N-terminus of IgG1 Fc for targeting of the avb3 integrin receptor. In other studies, enzyme catalyzed sortase mediated ligation has been utilized to produce Fc-BPI conjugates to suppress experimental autoimmune encephalomyelitis (EAE), a model disease induced in mice similar to multiple sclerosis.

