Research Overview—
The Schöneich research group is focusing on the detection and characterization of covalent oxidative post-translational modifications of proteins in vivo and in vitro. We are interested in the mechanisms by which these oxidative modifications form in tissues and in pharmaceutical formulations, and their biological or pharmaceutical impact.
Protein oxidation and nitration in vivo
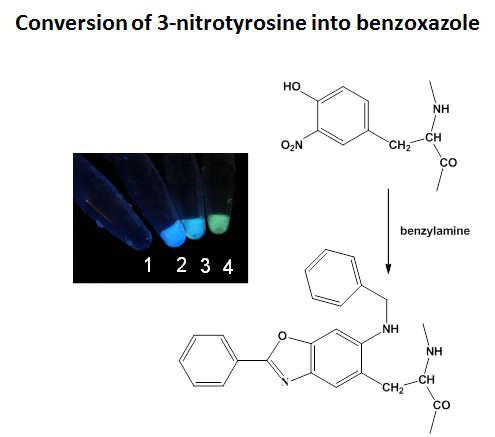
In vivo, protein oxidation is a hallmark of oxidative stress and accompanies many pathologies and biological aging. The oxidative modification of specific amino acid residues in proteins may lead to loss or gain of function, changes in protein conformation and turnover and/or protein-protein interactions. It is our goal to identify proteins, which contain modified amino acids, and to quantitate to what extent such modifications contribute to disease and/or aging phenotypes. We use high-resolution chromatography coupled to mass spectrometry (MS), as well as MS2 and MS3 analysis for the identification and characterization of post-translational protein modifications. In addition, we have developed a fluorogenic chemical derivatization method which converts both 3,4-dihydroxyphenylalanine (DOPA) and 3-nitrotyrosine into highly fluorescent benzoxazole derivatives through reaction with benzylamines. Depending on specific substitution patterns on the benzylamines, the emission maxima of the benzoxazoles vary, resulting in different colors, which are visible after protein derivatization in test tubes. We are currently applying the derivatization chemistry for the relative quantitation of potential biomarkers in neurodegenerative diseases. In addition, the fluorogenic derivatization of DOPA may serve for the rapid screening of the chemical stability of protein formulations.
Oxidative degradation of protein pharmaceuticals
Oxidation represents a major degradation pathway of protein pharmaceuticals. Oxidative protein degradation may lead to the loss of potency, and covalent cross-linking, potentially leading to aggregates. In addition, the chemical generation of new epitopes (neo-epitopes) on pharmaceutical proteins may contribute to immunogenicity. Therefore, a detailed characterization of protein oxidation products in pharmaceutical formulations is necessary, and is the goal of our research. For this, we use high-resolution chromatography coupled to mass spectrometry (MS), as well as MS2 and MS3. New instrumentation allows us to resolve protein degradation products on HPLC columns as long as 1.15 m, and with this technique we have isolated multiple isobaric and isomeric reaction products, which have escaped separation and analysis on conventional HPLC columns. As representative example for the types of new degradation products, we detected the light-induced conversion of tryptophan into glycine at very specific positions in a monoclonal antibody, and also disulfide isomerization products. Ongoing projects focus on the thorough characterization of oxidative and photo-induced degradation products in various monoclonal antibodies, the effects of subclass and glycosylation, as well as formulation parameters. For mechanistic studies, and to confirm product identity by complementary methods such as NMR, we synthesize model peptides, and evaluate their oxidative and photo-induced degradation pathways.
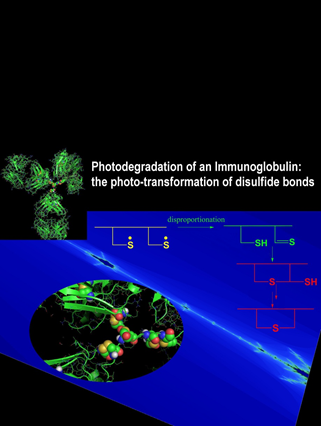
Chemical Research in Toxicology 2010, 23, 1310-1312
Chemical and physical stability of antibody-drug conjugates
We have started to evaluate the effect of drug conjugation on the physical and chemical stability of antibody-drug conjugates. In a first publication, we addressed light-induced degradation on a model antibody-drug conjugate, and detected that conjugation of a molecule with photo-sensitizing properties led to very efficient aggregation and chemical degradation. Currently, we are focusing on the effects of conjugates, which have no photo-sensitizing properties

